The Global Diagnostic Testing of Stds Market has become an essential segment in healthcare, focusing on the accurate and timely detection of sexually transmitted diseases (STDs) to prevent their spread and support effective treatment. With the increasing prevalence of infections such as chlamydia, gonorrhea, syphilis, and HIV, healthcare systems worldwide are placing a heightened emphasis on diagnostic testing. The market encompasses a range of testing technologies, including nucleic acid amplification tests (NAATs), enzyme-linked immunosorbent assays (ELISA), rapid point-of-care tests, and immunofluorescence assays, designed to offer rapid, precise, and cost-effective diagnosis.
Market Overview
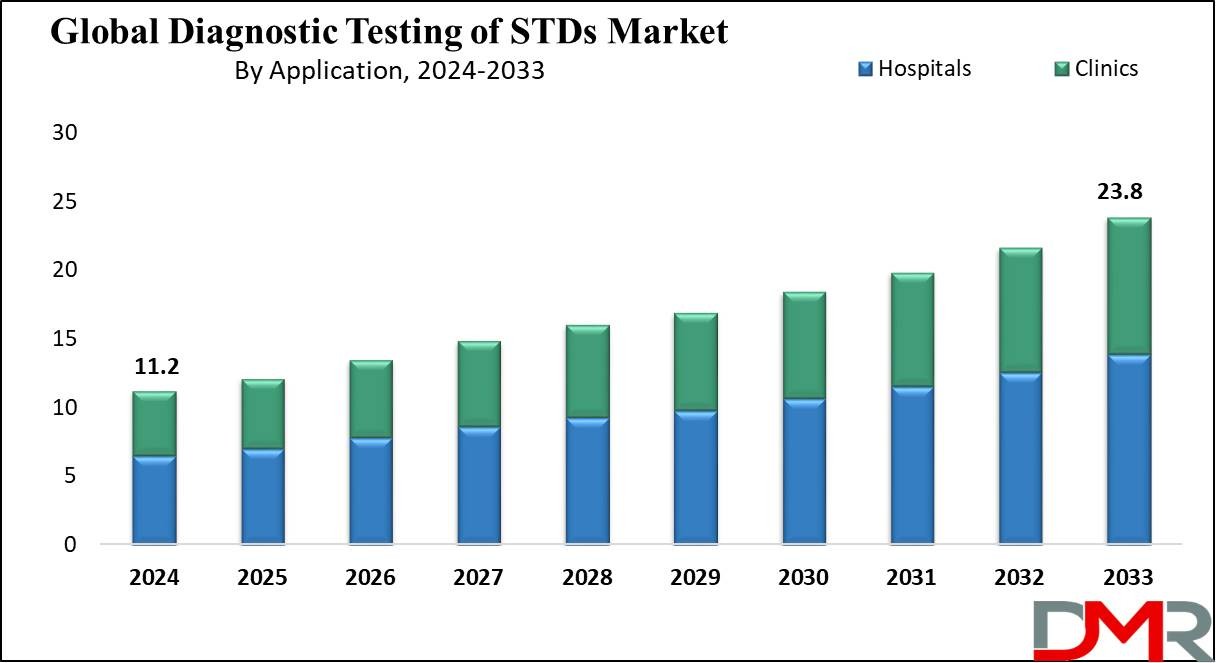
The global diagnostic testing of STDs market was valued at USD 11.2 billion in 2024 and is projected to reach USD 23.8 billion by 2033, registering a compound annual growth rate (CAGR) of 8.4% during the forecast period. The growing incidence of sexually transmitted infections, increased public awareness, and the expanding availability of advanced diagnostic tests are key factors propelling market growth. Rising government initiatives for STD prevention, robust funding for sexual health programs, and the introduction of innovative, rapid testing solutions have significantly contributed to market expansion.
Diagnostic testing plays a pivotal role in early detection and management of STDs, reducing complications, preventing long-term health issues, and controlling disease transmission. The development of highly sensitive and specific testing platforms ensures accurate detection of infections at early stages, which is critical for patient outcomes and public health. Additionally, the integration of diagnostics with telehealth and digital platforms is emerging as a strategy to enhance accessibility and patient engagement.
Market Drivers
Several factors are driving the growth of the diagnostic testing of STDs market. Firstly, the rising prevalence of STDs across all age groups, coupled with changing sexual behaviors and increased urbanization, has increased demand for efficient diagnostic solutions. Secondly, healthcare providers are increasingly focusing on early detection and preventive care, creating a robust market for advanced testing technologies.
Technological advancements in testing methodologies, including the development of multiplex assays, high-throughput screening, and rapid point-of-care devices, are further accelerating market growth. Governments and healthcare organizations worldwide are actively promoting STD screening programs to curb infection rates, improve sexual health awareness, and reduce the burden on healthcare systems. Public-private partnerships, strategic collaborations, and educational campaigns have contributed to heightened demand for diagnostic tests.
Market Restraints and Challenges
Despite significant growth, the diagnostic testing of STDs market faces certain challenges. Limited awareness and social stigma associated with STDs in some regions may hinder individuals from seeking testing services. High costs associated with advanced diagnostic kits and laboratory infrastructure can restrict adoption, particularly in low- and middle-income countries.
Regulatory challenges, including stringent approvals and varying standards across regions, may delay product launches and market entry. Additionally, integrating diagnostic testing into existing healthcare frameworks and ensuring data privacy and accuracy remain ongoing challenges. Variability in test sensitivity and specificity can also impact clinical decision-making, emphasizing the need for continuous technological advancements and quality control.
Technological Advancements
Technological innovation is a significant growth driver for the diagnostic testing of STDs market. Nucleic acid amplification tests (NAATs) are increasingly favored for their high sensitivity and ability to detect low pathogen loads. Rapid point-of-care testing devices enable immediate diagnosis in clinical and remote settings, facilitating timely treatment and reducing disease transmission.
Multiplex testing platforms allow simultaneous detection of multiple pathogens from a single sample, improving efficiency and patient convenience. Automation in laboratory workflows and high-throughput testing systems enhances productivity, reduces human error, and ensures standardized results. Additionally, advancements in molecular diagnostics, biosensors, and immunoassays are providing clinicians with accurate and reliable testing solutions.
Applications Across Healthcare
Diagnostic testing of STDs is applied across various healthcare settings, including hospitals, clinics, diagnostic laboratories, and community health centers. Routine screening and early detection programs target sexually active populations, high-risk groups, and pregnant women to prevent vertical transmission. Hospitals and laboratories leverage advanced testing platforms for accurate diagnosis, treatment planning, and patient management.
Community-based testing initiatives and mobile health units expand accessibility in underserved regions, increasing awareness and promoting timely testing. Rapid testing and self-testing kits empower individuals to monitor their health privately and discreetly. Insurance providers and public health programs increasingly support STD testing as part of preventive healthcare packages, driving adoption across diverse populations.
Market Segmentation
The diagnostic testing of STDs market can be segmented by test type, sample type, end-user, and region. Test types include nucleic acid amplification tests (NAATs), immunoassays, rapid tests, culture-based methods, and other molecular techniques. NAATs are preferred for their high sensitivity and specificity, while rapid tests offer convenience and immediate results. Sample types include blood, urine, swabs, and other biological specimens, with urine and swabs gaining preference due to non-invasive collection methods.
End-users encompass hospitals, diagnostic laboratories, clinics, public health institutions, and research organizations. Hospitals and laboratories account for the largest market share due to their advanced infrastructure, skilled personnel, and capacity to conduct high-volume testing. Public health programs and clinics play a crucial role in community screening and preventive care.
Regional Analysis
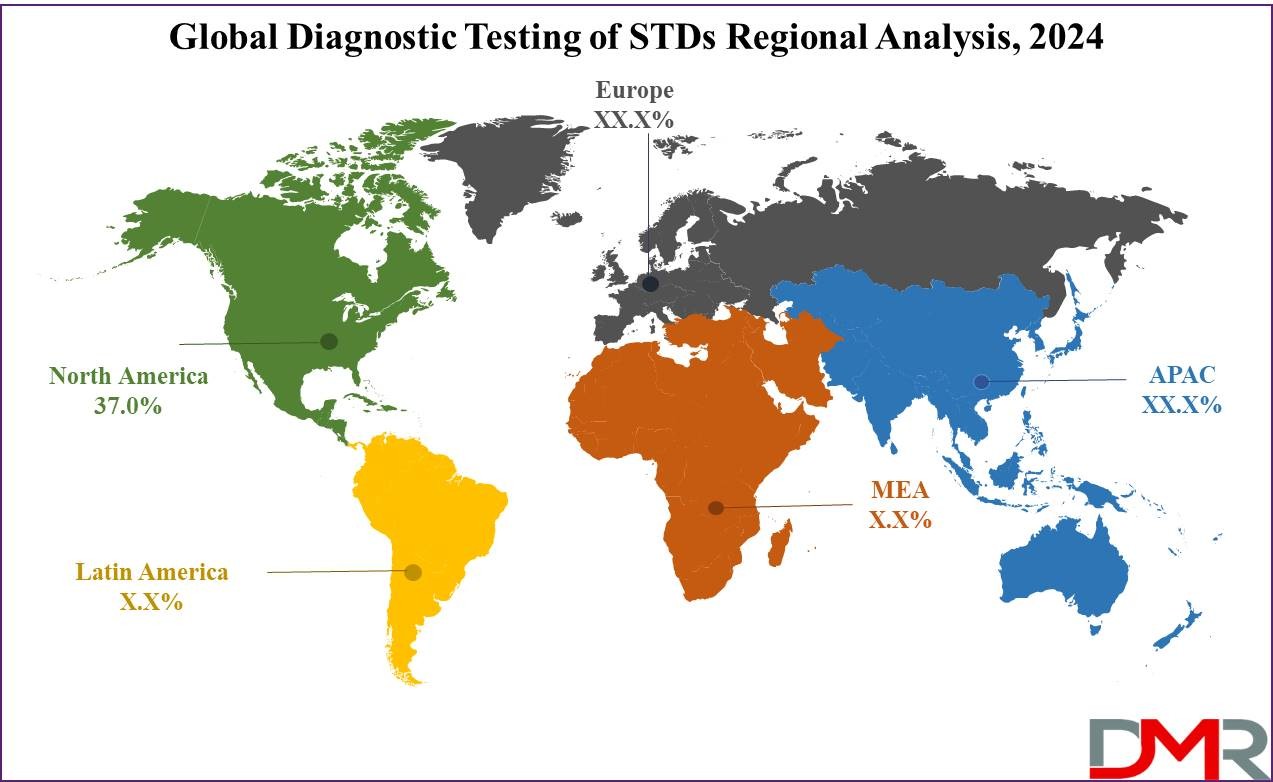
North America is anticipated to lead the diagnostic testing of STDs market throughout the forecast period, driven by the introduction of innovative test kits, high healthcare spending, and supportive government initiatives. The U.S. has a well-established healthcare infrastructure and active STD prevention programs, which drive demand for accurate and rapid diagnostic testing solutions.
Europe follows as a significant market due to rising awareness, adoption of advanced testing technologies, and strong healthcare frameworks. Asia Pacific is expected to witness substantial growth, fueled by increasing healthcare access, rising STD prevalence, and growing government initiatives for sexual health awareness. Latin America, the Middle East, and Africa are gradually investing in healthcare infrastructure and STD testing programs, presenting emerging opportunities for market expansion.
Download a Complimentary PDF Sample Report: https://dimensionmarketresearch.com/report/diagnostic-testing-of-stds-market/request-sample/
Competitive Landscape and Strategic Initiatives
The diagnostic testing of STDs market is highly competitive, with major players emphasizing innovation, strategic partnerships, acquisitions, and geographic expansion. Companies focus on developing advanced diagnostic platforms, multiplex testing solutions, and rapid point-of-care devices. Collaborations with hospitals, research institutions, and public health programs enhance market penetration and product adoption.
Market participants invest in research and development to improve test accuracy, reduce costs, and integrate emerging technologies such as AI-driven diagnostics and digital reporting. Regulatory compliance, quality control, and product standardization are critical competitive strategies. Additionally, partnerships with government programs and NGOs help expand testing initiatives in low- and middle-income countries, addressing unmet medical needs.
Emerging Trends and Future Outlook
Emerging trends in the diagnostic testing of STDs market include the integration of digital health platforms, telemedicine, and mobile applications to facilitate remote testing and result delivery. Self-testing kits and home-based diagnostics are gaining popularity, empowering individuals to monitor their sexual health privately.
Advancements in molecular diagnostics, biosensor technologies, and multiplex platforms are enhancing test sensitivity, specificity, and efficiency. Governments and healthcare organizations are increasingly implementing awareness campaigns, preventive screening programs, and subsidized testing initiatives to curb STD prevalence. With rising demand for early detection, technological innovations, and supportive policies, the diagnostic testing of STDs market is poised for significant growth, transforming sexual healthcare delivery and improving population health outcomes through 2033.
Frequently Asked Questions (FAQs)
What is the diagnostic testing of STDs, and why is it important?
Diagnostic testing of STDs involves detecting sexually transmitted infections through laboratory and point-of-care tests. It is essential for early detection, effective treatment, and prevention of disease transmission.
Which region holds the largest share in the STD diagnostic testing market?
North America is expected to lead the market due to the high introduction of innovative test kits, advanced healthcare infrastructure, and active government initiatives promoting STD testing.
What are the major drivers of market growth?
Key drivers include the rising prevalence of STDs, increased awareness of sexual health, technological advancements in diagnostics, government initiatives, and the adoption of rapid and multiplex testing platforms.
What are the common diagnostic tests used for STDs?
Common tests include nucleic acid amplification tests (NAATs), immunoassays, rapid point-of-care tests, culture-based methods, and molecular diagnostics for pathogens such as chlamydia, gonorrhea, syphilis, HIV, and hepatitis B and C.
What challenges does the market face?
Challenges include social stigma, limited awareness in certain regions, high testing costs, regulatory hurdles, integration with healthcare systems, and the need for quality control and data privacy compliance.
Summary of Key Insights
The global diagnostic testing of STDs market is poised for substantial growth, driven by rising STD prevalence, technological advancements, and increased awareness of sexual health. North America leads the market, supported by innovative test kits, strong healthcare infrastructure, and active government initiatives. Emerging regions in Asia Pacific, Latin America, and Africa present growth opportunities through increased healthcare access, preventive screening programs, and adoption of rapid testing solutions. The integration of digital health platforms, multiplex diagnostics, and self-testing kits is shaping the future of STD diagnostics, improving accessibility, accuracy, and population health outcomes worldwide.
Purchase the report for comprehensive details: https://dimensionmarketresearch.com/checkout/diagnostic-testing-of-stds-market/



